But copper is more dense, and therefore has more mass per unit volume. Our applications are generally limited by volume, and the heat capacity is actually about the same
per unit volume.
A heatsink made of copper will have a greater mass than an aluminum heatsink the same size. Weather or not that difference in mass is significant depends on the size of the heatsink. True, if you are staying small enough the difference may not be as much but there will be a point where copper would have more thermal capacity per unit volume than aluminum. When I get home I will pull up a few heatsink models and post the mass of certain heatsinks in aluminum and copper. (say a C6 aluminum and copper heatsink.) I believe that even in a heatsink so small the difference in mass between aluminum and copper is pronounced enough that the copper heatsink will still have a higher overall thermal capacity.
You mean to say an alloy of aluminum is half as conductive as pure aluminum? That's a rather large difference for such a small change in material. Source?
I don't have a link for each source, but if you google thermal conductivity of aluminum **** you should get MSDS results that will confirm what I am saying.
The thermal conductivity of 6061, the material most likely to be used is 160W/mK, 6063 (another candidate, more expensive but better to anodize.) is 200W/mK. 7075, a structural aluminum probably not used in lasers much due to cost is 130W/mK. These numbers will change depending on the temper of the aluminum as well.
That's a bit misleading since the heat isn't created all at once. If you actually take the temperature of various parts of aluminum, it's virtually all the same. Take this image of the arctic for example. The junction of the tail cap provides FAR more resistance than even several inches of aluminum. If it were made of copper, the image would not be much different
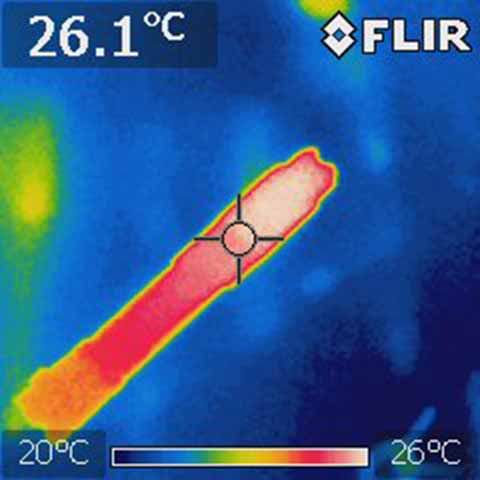
This picture confirms what I am saying is true. There is a clear difference in temperature between the head and body of the host so the temperature between one material (aluminum here) is not the same all the way through. Sure, it's not much but it is there. If the arctic was made of copper the difference in temperature would be less pronounced.
Also, the heat is created all at once. As soon as that diode turns on it is wasting energy, that energy is distributed through the host as heat. As long as the diode is running at a constant current it will make (close to) the same amount of waste heat every second.
A video of the arctic running from room temperature would show similar results, the head would get hot before the body did. If you used copper the body would heat up faster than the aluminum since copper's thermal conductivity is that much higher than aluminum.




