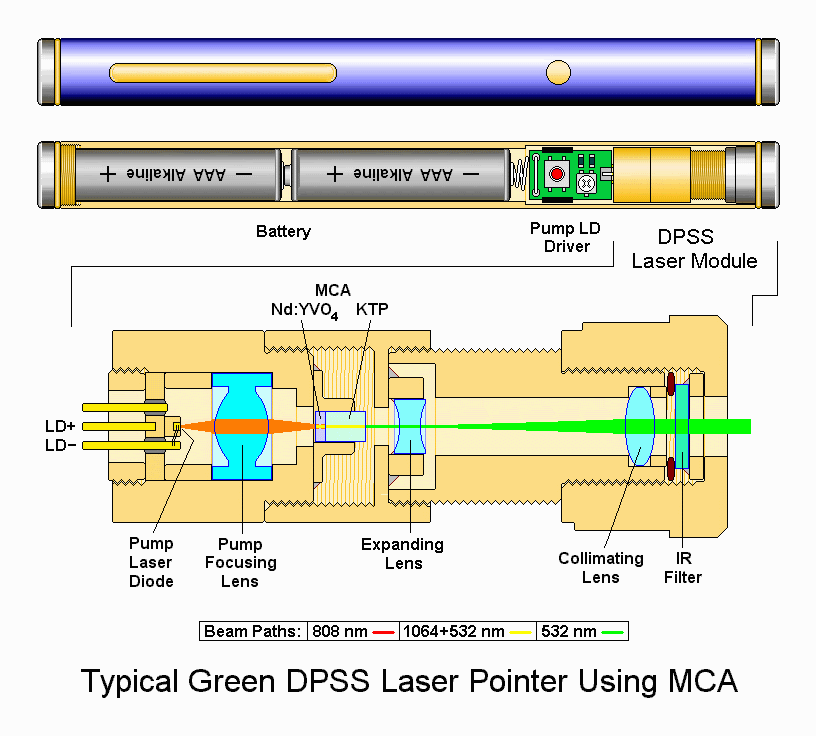
- Joined
- Jul 11, 2009
- Messages
- 11
- Points
- 0
i'm trying to get a handle on how each color laser is constructed, and their various strengths and weaknesses, to make a choice on my first diy build. any input would be welcome.
this is what i've learned so far:
~405nm: violet color, diodes available in br burners, great burners, fluorescence
~473nm: blue color, obtained by lengthening the wavelength of a 405nm using crystals, rare unique color, not great at burning or terribly bright
~510nm: green color, obtained by shortening the wavelength of 700nm+ infrared diodes, most brilliant color to the human eye so great brightness, not a great burner
~570nm-590nm: yellow through orange colors, obtained by altering some other native wavelength diode (?), unusual colors, not great burners, not as bright as green
~650nm: red color, most common diode, not as bright as green, not a great burner
>700nm: infrared invisible beam, diodes available in dvd burners and for direct purchase, hard to work with and dangerous because you can't see the beam, not a good burner
anywhere i've got the wrong idea, or am leaving out something important about a particular wavelength, let me know. i realize burning is a difficult way to summarize a wavelength since there are so many other factors, but i am just comparing basically one wavelength of each color against each other.
is it possible to purchase diodes that emit wavelengths other than violet, red, and ir? or is every other color solely available through precision alignment of crystals that change one of those three wavelengths?
thanks in advance for any advice or input!
this is what i've learned so far:
~405nm: violet color, diodes available in br burners, great burners, fluorescence
~473nm: blue color, obtained by lengthening the wavelength of a 405nm using crystals, rare unique color, not great at burning or terribly bright
~510nm: green color, obtained by shortening the wavelength of 700nm+ infrared diodes, most brilliant color to the human eye so great brightness, not a great burner
~570nm-590nm: yellow through orange colors, obtained by altering some other native wavelength diode (?), unusual colors, not great burners, not as bright as green
~650nm: red color, most common diode, not as bright as green, not a great burner
>700nm: infrared invisible beam, diodes available in dvd burners and for direct purchase, hard to work with and dangerous because you can't see the beam, not a good burner
anywhere i've got the wrong idea, or am leaving out something important about a particular wavelength, let me know. i realize burning is a difficult way to summarize a wavelength since there are so many other factors, but i am just comparing basically one wavelength of each color against each other.
is it possible to purchase diodes that emit wavelengths other than violet, red, and ir? or is every other color solely available through precision alignment of crystals that change one of those three wavelengths?
thanks in advance for any advice or input!