Thermal conductivity without electrical conductivity, tough to do.
How does heat move through a material?
Well, the most common is through electron transport, hot electrons diffusing into cold areas. This is what metals use, since they have a sea of electrons that can all move. The catch there? Yep, electrons moving means electrical conductivity as well as thermal.
What's the next most common? Phonon transport. A phonon is a lattice vibration, but it can travel through interconnected networks, such as an atomic lattice. Temperature is average kinetic energy of all the atoms in a solid, and that kinetic energy is in the form of each atom vibrating in it's slot. Imagine a bar of, say, diamond, which doesn't conduct electrons. Now say one side is hotter than the other. Which means the atoms on that side are vibrating at higher energy and amplitude. Since all the atoms are connected together in a network, that vibration can be transferred through the material to the atoms that are colder, and eventually, all the atoms will be vibrating at the same energy/frequency. The action of that vibration moving through a solid mimics that of a particle moving through the material, and that imaginary particle is called a phonon. (Another visual aid: imagine a huge 3-D networks of spheres all connected to their neighbors with springs, forming cubes. Now, thump one of those spheres, and observe the vibration moving through the network. That is a phonon.)
So, you need good phonon transport. Turns out, any imperfection in a material will inhibit phonon transport. Grain boundaries, dislocations, everything. Things will still transport phonons, but not very efficiently. So it's really hard to do. When using paste or something like that, you still have to rely on the vibrations of atoms, but the less perfect a network is, the worse is it.
For something like Arctic silver, they put actual silver in the paste. The heat can move through the small bits of metal in the paste, but can't overall conduct because the electrons can't jump from one silver particle to the next. But the charge will still move within the particles, and having this charge in there and having it close to electrical vias and paths, the paste can act in a capacitive fashion, which can screw with the electrical components even if it won't conduct and act like a short.
There is one other big way that heat can conduct, but surely we all know that one, given the kind of forum we're all on.



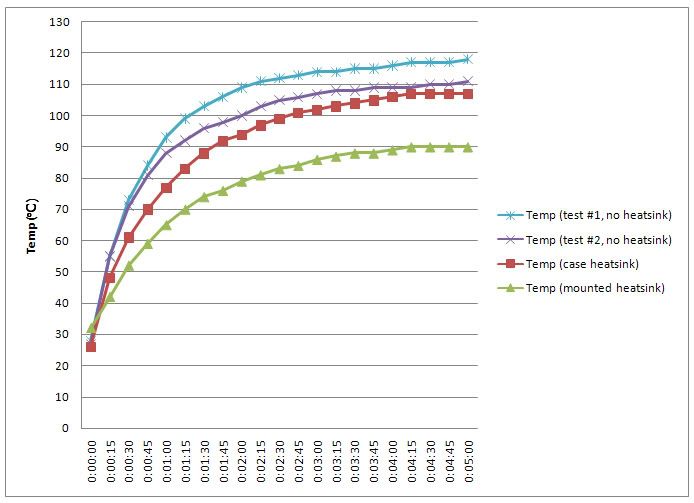
IIRC around 4x as thermally conductive as copper (silver is a tiny bit better than copper)....


