First I would like to give credit to DTR, Jayrob and all the other LPF contributors whose posts I have read. :thanks::bowdown:
I would not have been able to do this with out copying them quite a bit (I hope that is ok?). I am new to building lasers so please pardon my mistakes and I would be happy to hear any suggestions. I am sure someone has thought of this before me.

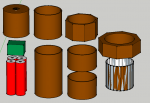
Please see the added picture made using Google's Sketchup (btw sketchup is free, easy and pretty powerful 3D modeling software)
From left to right and top to bottom these are the pieces in the picture:
1st Column: Heatsink, FlexMod3 Driver, Four 18650 protected batteries
2nd: the two sections of copper pipe that will be joined with the coupling
3rd: two coupling pieces with the end cap below
4th: Coupling Nut, Union possibly shortened and used between the cap and coupling to thicken the pretty thin pipe in that area (the union looks weird I think because Sketchup has trouble rendering it because it's so thin?).
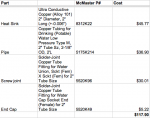
My idea is to use a copper pipe for a laser host. A solid copper rod would be used for the heat sink with a hole in the middle for the module. The heat sink, coupling and cap will be soldered to the pipe. The module will either be press fit into the heat sink or held in place with a set screw. The coupling on the pipe will be set just below the heat sink to allow access to the driver, module and battery charging. The batteries will be set up as two sets of two batteries in series then those two series sets in parallel. I plan on putting a flush mount side clicky near where the driver is. I might coat it with varnish to keep it from oxidizing.
This thing will be pretty massive but I have huge hands anyways and I want something with plenty of power, heat sinking and room so I can modify it over time.
If and when I do build this I will probably do it slowly finishing before Christmas as my first kid is due in December and I just got a new job which take priority.

I would not have been able to do this with out copying them quite a bit (I hope that is ok?). I am new to building lasers so please pardon my mistakes and I would be happy to hear any suggestions. I am sure someone has thought of this before me.
Please see the added picture made using Google's Sketchup (btw sketchup is free, easy and pretty powerful 3D modeling software)
From left to right and top to bottom these are the pieces in the picture:
1st Column: Heatsink, FlexMod3 Driver, Four 18650 protected batteries
2nd: the two sections of copper pipe that will be joined with the coupling
3rd: two coupling pieces with the end cap below
4th: Coupling Nut, Union possibly shortened and used between the cap and coupling to thicken the pretty thin pipe in that area (the union looks weird I think because Sketchup has trouble rendering it because it's so thin?).
My idea is to use a copper pipe for a laser host. A solid copper rod would be used for the heat sink with a hole in the middle for the module. The heat sink, coupling and cap will be soldered to the pipe. The module will either be press fit into the heat sink or held in place with a set screw. The coupling on the pipe will be set just below the heat sink to allow access to the driver, module and battery charging. The batteries will be set up as two sets of two batteries in series then those two series sets in parallel. I plan on putting a flush mount side clicky near where the driver is. I might coat it with varnish to keep it from oxidizing.
This thing will be pretty massive but I have huge hands anyways and I want something with plenty of power, heat sinking and room so I can modify it over time.
If and when I do build this I will probably do it slowly finishing before Christmas as my first kid is due in December and I just got a new job which take priority.
Attachments
Last edited: