- Joined
- Sep 12, 2007
- Messages
- 9,399
- Points
- 113
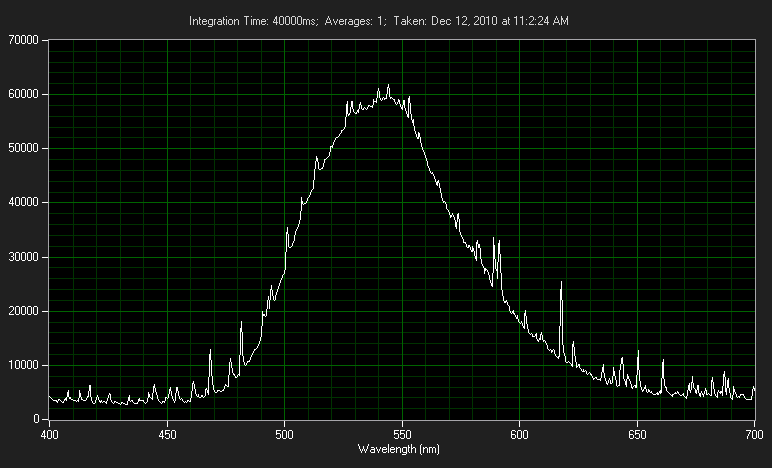
The output is not really monochromatic like a laser, but not an overly broad spectrum either. It could be comparable to that of a colored LED.
It is MUCH broader than an LED. It is very close in color/spectrum to glow-in-the-dark material.
Here is a spectrograph I took of my 6 inch vial a while back. The spikes are just noise from the 40-second exposure that was required.
Last edited:



