- Joined
- Aug 25, 2007
- Messages
- 2,007
- Points
- 63
:wtf: How do you find the colour of light from the frequency??
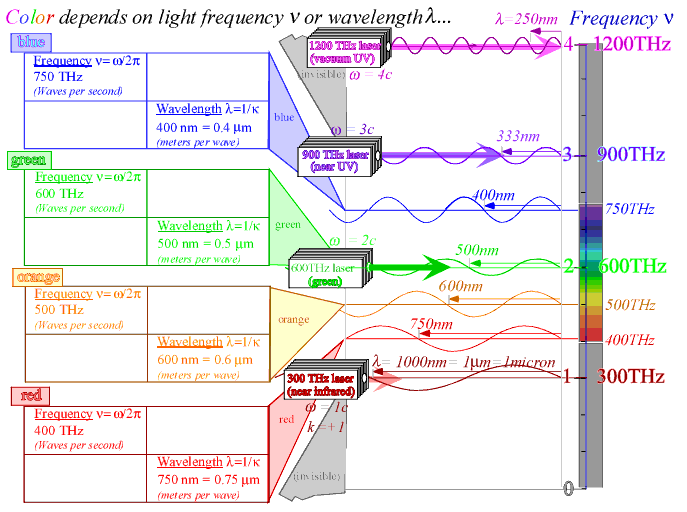
That's what I've been trying to tell you. (Wavelength) * (frequency) = speed, since speed is a constant in air, wavelength and frequency are inextricably linked. The speed of light in vacuum/air is ~3(10)^8 m/s, always, for all wavelengths. So speed divided by wavelength is frequency. Frequency is exactly as good as wavelength, because we're operating in a regime where n=1. Since n=1, and the speed of light is constant, wavelength and frequency are inextricably linked. Frequency is just as good at indicating color as wavelength is.