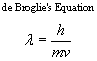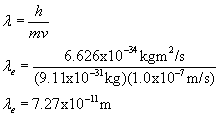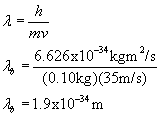Re: Not laser related, but a great thinking exerci
One of my favorite cartoons is Futurama. In one episode the professor and the crew are at the race track betting on the horses. At the finale, two horses cross the finish line together, so close in fact it's too close to call. The announcer calls over the loudspeaker that the winner was Lasty in a quantum finish. The professor screams out, "No fair, you changed the outcome by measuring it!" I think the cartoon was too cerebral for average Joe Citizen.
Anyway, the Heisenberg uncertainty principle is just freakin cool. If you are measuring a particle for example there is no way you can know the momentum and the position at the same time. If you measure the position you have essentially snapshotted an instantaneous moment in time. By taking that snapshot you destroy any information about it's motion. Likewise by measuring momentum you must have a timescale. By having a timescale to measure the momentum you cannot know where the particle is in that time period.
An example is electron motion around an atom. We have been raised with the image of electrons in orbits around the nucleus like the planets around the sun when in fact electron motion is a probability equation that determines the probability of an electron being in a given location at a given time. What happens is that an electron cloud develops with an electron appearing here then there with no defined orbit only the probability of it being here or there.
It's freaky. It also drove Einstein nuts. He was a right angle man, meaning everything had an exact solution whereas quantum physics included probability and chance. He did come to accept it later in his life but his famous statement, "God does not play dice." is often used to define the man. Hawking later played on this by stating, "God not only plays dice, but sometimes throws them where they cannot be seen."
Of course we do not see this on a macro scale even though it is happening. Someone mentioned the collapse of the wave function on an observation which is what happens to quantum physics when applied to the macro world. Waves play a huge role in quantum mechanics but we cannot detect waves from a tree or a person. They are there however but the wavelength is so short and the quantum effects so small that it is lost on the macro world.
MODIFY:
Alright I calculated a demonstration to show the differences in wavelength. Took me a second to turn my equations into images but here they are.
The de Broglie equation can be used to calculate the wavelength of an object in motion.
I've calculated an electron (mass 9.11E-31kg moving at 1E7 m/s) and a sphere (mass .10kg moving at 35 m/s)
ELECTRON
SPHERE
As you can see there are orders of magnitude difference in the wavelength of a quantum effected particle and a macro object. With such a short wavelength what we notice in the macro world is the particle nature of matter.